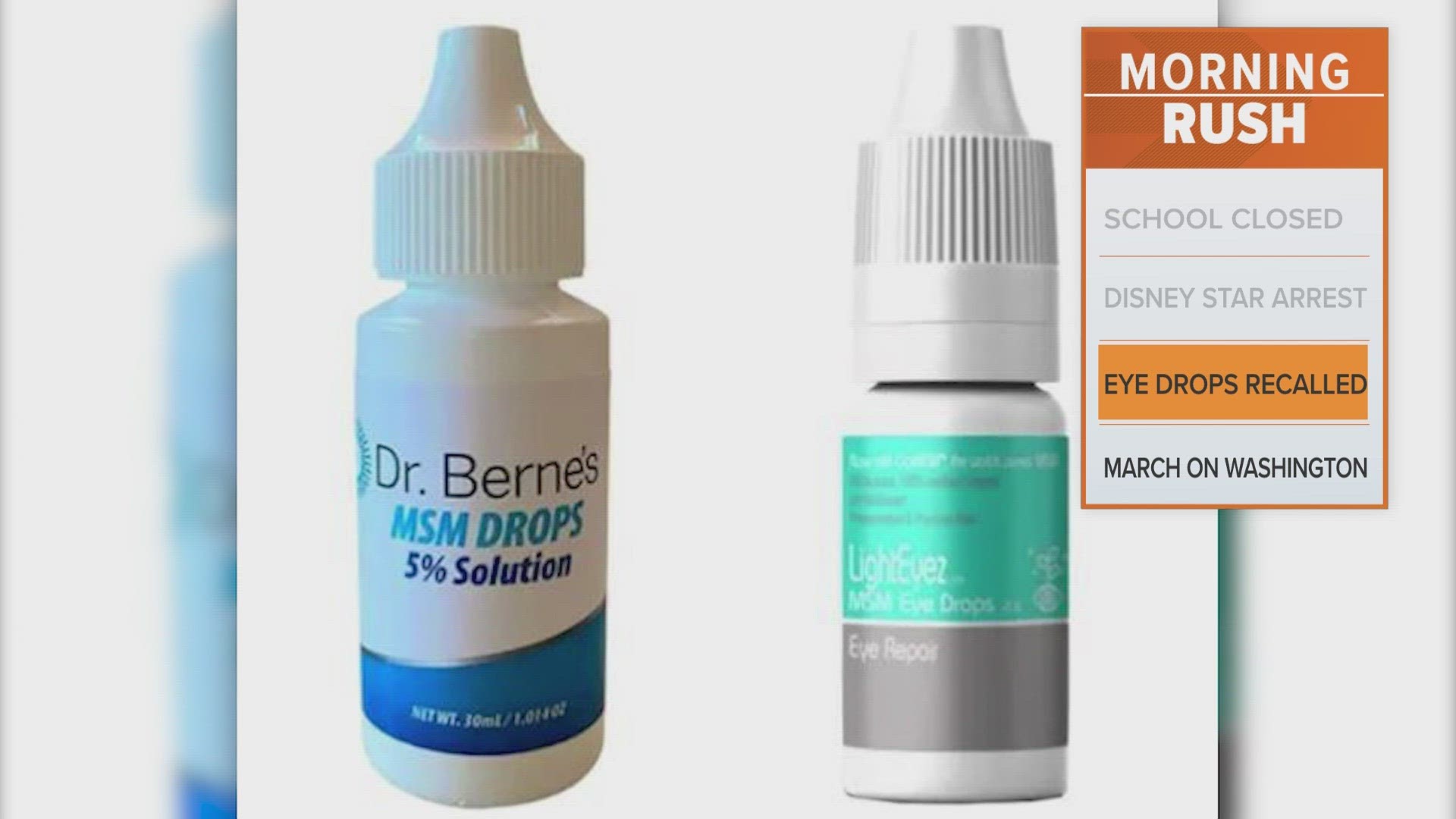
WASHINGTON — The FDA is warning consumers to stop using certain brands of eyedrops because they may be contaminated with bacteria, fungus or both; the eyedrops also contain unapproved drugs that aren't legally marketed in the U.S.
The warning is for "Dr. Berne's MSM Drops 5% Solution" and "LightEyez MSM Eye Drops - Eye Repair" products, according to the U.S. Food and Drug Administration.
The products are distributed by Dr. Berne's Whole Health Products and LightEyez Limited, respectively.
The eyedrops contain methylsulfonylmethane, often shortened to MSM, as an active ingredient. MSM is unapproved in the U.S. as an eye drug, and the FDA says the eye drops were illegally marketed.
Additionally, when testing samples of the two eyedrop brands, the FDA found that they were contaminated with microbes and weren't sterile. Under federal law, eye drops must be sterile to be safe for use on humans.
Five different strains of bacteria were found between the two eye drop products. Additionally, the Dr. Berne's drops had fungal contamination as well, health officials said.
The FDA is urging all users to check their eye drops and throw them away if they're among the affected products. Shoppers should also avoid those products in stores.
Dr. Berne officials told the FDA on Aug. 21 they would voluntarily recall the MSM drops. The company announced Saturday a voluntary recall for all Dr. Berne's MSM Drops 5% Solution, 15% Solution, Dr. Berne’s Organic Castor Oil Eye Drops and Dr. Berne’s MSM Mist 15% Solution.
The company said it has received two reports of adverse events related to this recall, although the recall notice did not specify how severe the cases were.
The FDA said it has been unable to reach LightEyez Limited, and the federal organization says the company has not responded to emailed messages trying to discuss the safety concerns.
"To date, LightEyez has not responded to FDA or taken action to protect consumers," the FDA wrote in a statement.
Using contaminated eye drops can lead to serious infections, which could threaten both a user's vision and life.
Anybody with signs of an eye infection after using either product should seek medical help immediately. Anyone who experiences an adverse reaction from either eye drop product can submit a complaint to the FDA’s MedWatch Adverse Event Reporting program.